LIVER DAMAGE IN EUROPE ASSOCIATED WITH HERBALIFE USE:
4 Sept 2007 ReviewThe following reports below of HerbaLife-associated liver failure appear on Medline – from Spain, then Israel and Switzerland.
:The July 2007 reports of the two dozen Herbalife-associated hepatitis cases from Israel & Switzerland reveal that liver problems occurred after about 5 months on the products; and that relapse occurred in about 20% on rechallenge with Herbalife ie in this percentage the association is proven.
Herbalife results for weight control have been reported as good. The only problem is historical according to the current Wikipedia entry: “Some of the original Herbalife weight loss products contained the active ingredient Ma Huang or Sida cordifolia, two herbs containing ephedrine alkaloids.
Adverse reactions involving the company’s Thermojetics original green tablets were recorded by the U.S. Food and Drug Administration and Herbalife subsequently stopped using ephedrine in its products in the face of rising insurance premiums.[3][4] The U.S. FDA banned supplements containing ephedra in 2004.[5]“
It is possible that the case reports below are unrelated to Herbalife itself , or that in those countries ephedra-containing Herbalife was still in use at the time, or that potentially hazardous herbs etc were added locally.
From Yahoo.com, there is an authoritative rebuttal from Iceland dated February 2007.
A score of drugs and herbs can cause liver damage, topical ones – albeit rarely- include mushrooms; black cohosh and kava – see a recent list.
Drugs like ticrynafen, methyldopa and cerivastatin were discarded among other reasons because of liver problems, which are among many reasons why necessary sex hormone contraception and replacement should rather not use designer patent ie synthetic drugs, and especially not by mouth (hepatic first pass effect).
So it is always difficult to blame a single product, as the ongoing debate about black cohosh shows – which many “first world” regulators have “black boxed” ie added a compulsory warning to black cohosh warnings.
As with black cohosh, with a rare adverse event report, users of such products must weigh up for themselves.
As they say, since Herbalife tends to be custom-made in each country, with numerous ingredients (some undisclosed), it is so far impossible to incriminate whether the cause was local product corruption, or some appreoved component, of which the known possible culprits are ephedra and camelia.
Other known hepatotoxic herbs like black cohosh, kava and mushrooms were not mentioned. A few of the patients had viral hepatitis. Only 7 cases had also taken other known potential liver sensitizers – some synthetic sex hormones (4), aspirin (3), statin (1) and hydrochlorothiazide(1), of which 2 cases had positive recurrence of hepatitis on rechallenge with Herbalife.
UCT Medicines Information Centre is unaware of any such problems locally, and can recollect only perhaps 2 queries about Herbalife in some 23 years. Clarification is awaited from Herbalife headquarters.
From Swiss data the estimated incidence was below 2 cases per million Herbalife users, but both studies were based only on hospital records.
Considering the severe global problem of hepatitis from other causes (due to alcohol; obesity/diabetes (steatohepatitis, sulphonylureas, glitazones); numerous infections; carbon tetrachloride; synthetic sex hormones (oral contraception and postmenopausal hormone therapy) , mushrooms, antibiotics and antivirals, , autoimmune disease, antiepileptics, nifedipine, amitryptiline, allopurinol, nonsteroidal anti-inflammatories including aspirin and paracetamol , black cohosh, kava, antifungals and paracetamol), and that the rare adverse association of herbalife with liver damage may well have been limited only to Herbalife products made in those three “European” countries at that time, there is clearly no cause for alarm about Herbalife – just awareness.
The urgent problem of endemic liver disease is rather the avoidance of infections and potentially hazardous antimicrobials; mushrooms; carbon tetrachloride, alcohol excess; sale pf paracetamol without inclusion of protective vitamins and N-acetyl cysteine; and avoidance of potential hepatotoxins which are rarely if ever justified considering their risks, and safe effective alternatives available for eg statins, sulphonylureeas, glitazones, black cohosh, kava, non-steroidal anti-inflammatories; oral sex hormones; and sulphonylureas.
The centuries-proven plant galega officinalis (extract) metformin after 85years of modern use remains the only drug proven in longterm use to both reduce liver damage, lipidemia, thrombosis, adiposity and insulin resistance, and thus almost halve the incidence of new diabetes, hypertension/vascular disease, cancer and thus all-cause premature medical mortality.
Thus appropriate general use of metformin with long-proven vitamins, minerals, biologicals, safe herbs, fish oil and systemic human sex hormones – combined with prudent lifestyle and largely natural fresh foodstuffs- – does away with most of the well-known potential hepatitis drug risks listed above.
In defence of free market enterprise and choices, those who choose convenience safe proven food substitutes or other complementary products as part of an acceptable balanced regime advocated by suppliers like Herbalife do well, they should just be sure of the ingredients and supplier; and they should report and discuss what they use with some knowledgable up-to-date healthcare provider.
Response from Herbalife
04.09.07
Herbalife’s South Africa CEO responds reassuringly:
Good day,
Herewith a statement from Herbalife in response to the issues raised by yourself earlier in the week:
While we are aware of reports of abnormal liver function blood tests such as those reported by Dr. Oneta, our extensive consultation with internationally recognised liver experts has led repeatedly to the conclusion that these associations in time cannot be linked to any Herbalife product.
These small numbers of reports are anecdotal and millions of satisfied customers all over the world have been using our products for more than 27 years. All Herbalife products are formulated and manufactured in accordance with strict standards overseen by the Herbalife Scientific Advisory Board, which is chaired by David Heber, M.D., Ph.D., F.A.C.P., F.A.C.N. Quality control is overseen by our Scientific Affairs Group, chaired by Y. Steve Henig PhD and made up of an international panel of experts in nutrition and botanical dietary supplements.
Herbalife products, which are now sold in 65 countries, are formulated, registered and labelled in accordance with the regulatory requirements in every market where sold. All Herbalife products are safe to consume as directed.
Many consumers who choose to use Herbalife weight-management products for weight loss are overweight, some significantly so. Pre-existing medical conditions such as obesity and diabetes can be associated with non-alcoholic fatty liver disease, a disorder that may return certain types of abnormal blood test results. These test results, therefore, may have nothing to do with any herbal supplement, but rather are the result of a pre-existing medical condition. In addition, it is possible for an individual to have an allergic reaction to our products, the same way one might to any food product; for example, strawberries or shellfish. Herbalife supports the recommendation that consumers visiting their doctors for medical treatment inform them of any supplements they may be taking.
As a socially responsible company, we operate an adverse event reporting procedure that deals with the small number of queries we have from doctors and consumers and we operate an open dialogue policy with the medical community. All adverse event reports are investigated thoroughly in consultation with the consumer and the physician (if they are available) to fully understand the facts. None have resulted in the compulsory withdrawal of any product, ever. In the United States, Herbalife actively lobbied Congress to pass legislation mandating the submission of all dietary supplement and over-the-counter drug serious adverse events to the Food & Drug Administration. That new law takes effect December 22, 2007.”
REFS:
J Hepatol. 2007 Oct;47(4):521-526. Herbal does not mean innocuous: Ten cases of severe hepatotoxicity associated with dietary supplements from Herbalife((R)) products. Schoepfer AM, ea.University Hospital Bern, Switzerland.
METHODS: To determine the prevalence and outcome of hepatotoxicity due to Herbalife((R)) products. A questionnaire was sent to all public Swiss hospitals. Reported cases were subjected to causality assessment using the CIOMS criteria. RESULTS: Twelve cases of toxic hepatitis implicating Herbalife((R)) preparations (1998-2004) were retrieved, 10 sufficiently documented to permit causality analysis. Median age of patients was 51 years (range 30-69) and latency to onset was 5 months (0.5-144). Liver biopsy (7/10) showed hepatic necrosis, marked lymphocytic/eosinophilic infiltration and cholestasis in five patients. One patient with fulminant liver failure was successfully transplanted; the explant showed giant cell hepatitis. Causality assessment of adverse drug reaction was classified as certain in two, probable in seven and possible in one case(s), respectively. CONCLUSIONS: We present a case series of toxic hepatitis implicating Herbalife((R)) products. Liver toxicity may be severe. A more detailed declaration of components and pro-active role of regulatory agencies would be desirable.
J Hepatol. 2007 Oct;47(4):514-520. Association between consumption of Herbalife((R)) nutritional supplements and acute hepatotoxicity. Elinav E, ea -Hebrew University Medical Center, Israel.
: In 2004, identification of four index cases of acute hepatitis associated with Herbalife((R)) intake led to a ministry of health investigation in all Israeli hospitals. Twelve patients with acute idiopathic liver injury in association with consumption of Herbalife((R)) products were investigated.
RESULTS: Eleven of the patients were females, aged 49.5+/-13.4 y. One patient had stage I primary biliary cirrhosis and another had hepatitis B. Acute liver injury was diagnosed after 11.9+/-11.1 months of initiation of Herbalife((R)) consumption. Liver biopsies demonstrated active hepatitis, portal inflammation rich with eosinophils, ductular reaction and parenchymal inflammation with peri-central accentuation.
. CONCLUSIONS: An association between intake of Herbalife((R)) products and acute hepatitis was identified in Israel. We call for prospective evaluation of Herbalife((R)) products for possible hepatotoxicity.
Med Clin (Barc). 2007 Feb 17;128(6):238-9.
[Hepatotoxicity associated with the consumption of herbal slimming products] Duque JM,ea. [Article in Spanish] Letter
-----------------------------------
The C.A.M. Report
Complementary and Alternative Medicine:
Fair, Balanced, and to the Point
Liver toxicity associated with Herbalife dietary supplements
 Researchers from the University Hospital of Bern in Switzerland report 10 cases of hepatotoxicity.
Researchers from the University Hospital of Bern in Switzerland report 10 cases of hepatotoxicity.First, the details.
- A questionnaire was sent to all public Swiss hospitals.
- Cases covering the years 1998 to 2004 were evaluated to determine a causative role of the products.
- Herbalife dietary supplements were implicated in 12 cases of toxic hepatitis — 10 cases warranted further assessment.
- The time from starting treatment to onset of liver toxicity was 5 months (range 0.5-144).
- Liver biopsy in 7 cases showed hepatic necrosis (liver cell death).
- There was marked lymphocytic/eosinophilic (white blood cell) infiltration and cholestasis (blocked bile flow) in 5 patients.
- One patient with fulminant (severe) liver failure got a liver transplant.
The bottom line?
Herbalife International is a company that sells weight-loss, nutrition and skin-care products. The abstract does not specify which ones.
The authors’ abstract from Digestive Diseases Week meeting in 2006 concludes, this is the first case series of toxic hepatitis implicating Herbalife products.
- Mostly immuno-allergic mechanisms appear to be responsible.
- Liver toxicity may be severe, causing fulminant hepatic failure and veno-occlusive (blockage) liver disease.
- A high degree of suspicion and appropriate history taking is mandatory to reveal the potential for hepatotoxicity of “innocuous” dietary supplements.
- Also, better declaration of components and a more active role of regulatory agencies in surveillance of such products would be desirable.
This entry was posted on Tuesday, September 4th, 2007 at 11:07 PM
---------------------------
BACTERIA IN HERBALIFE DAMAGES LIVER
Two years ago liver doctors sounded the alarm about cases of liver damage
in users of Herbalife products. They were unable to say which component
in Herbalife products was causing the liver damage, but researchers at
the University of Bern made an attempt to do so in an article published
this year in the Journal of Hepatology. They found the bacteria Bacillus subtilis in Herbalife products.

|
In the article the doctors discuss two cases of people who became ill
after using Herbalife products: a man aged 78 and a woman aged 50. The
man's urine
had turned dark brown, he had hepatitis and had been feeling unwell for
a couple of weeks. He had been using the Herbalife F1 Shake [Strawberry
and Cappuccino flavours] for three years on his daughter’s advice [she
was a Herbalife salesperson], as well as various other medicines.
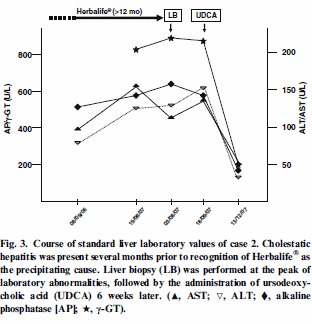
According to the analyses, the man had a liver complaint. When the
doctors took him off the shake his blood values recovered, but they
deteriorated again later. The doctors gave the man corticosteroids and ursodeoxycholic acid, after which he recovered completely. You can see the fluctuations in the man’s blood values in the graph below.

The woman sold Herbalife supplements. She took half a dozen different
Herbalife supplements, including the Personalized Protein Powder Mix
Formula 3. She had stomach pain
and hepatitis and the doctors found signs of liver damage in her blood.
They got the woman to stop taking the supplements and the graph below
shows how the liver values in her blood improved.
The researchers examined samples from the livers of the man and the
woman and found signs of damage in both. The doctors turned the
supplements that the men and women had used inside out, but found no
contaminants: no heavy metals, no pesticides, no antibiotics, nothing.
But when they examined the Herbalife products for micro-organisms, they
did come across something. The meal substitutes the man and woman had
been using contained the bacteria

|
Hmm.
Honestly speaking, we’re not sure what to make of this. As far as we know, B. Subtilis is found in pretty much everything, and it’s not particularly dangerous. [Wikipedia]
Some studies even regard it as a probiotic. It would seem pretty unlikely then that B. subtilis is the cause of such serious liver damage.


I value the blog.Thanks Again. Awesome.
ReplyDeletepenis enlargement